Section 2 Classification of the Elements Continued
Need for the Periodic Classification of Elements
All existing matter in our surroundings is made up of basic units known as elements. Initially, in 1800, only 31 chemical elements were discovered. After some advancement in technology in 1865, about 63 more elements were discovered. This created the need for the periodic classification of elements.
Presently, there are 118 elements known to us. Out of these 118 chemical elements, some elements are man-made.
- When there were only 31 elements it was relatively easy to study the properties of these chemical elements individually.
- Now the number of elements has raised to 118, it would be very cumbersome to study the properties of every element individual.
- In order to ease the work, scientists started thinking about a method so that the study of elements can be simplified.
- They decided to organize the elements in a periodic table according to the information available about the elements and various characteristics shown by them.
It was observed that elements show periodicity in their properties. Many tables were made to arrange the elements in an ordered manner based on their characteristics to study the properties of elements in a fixed pattern.
Periodic Classification of Elements Characteristics
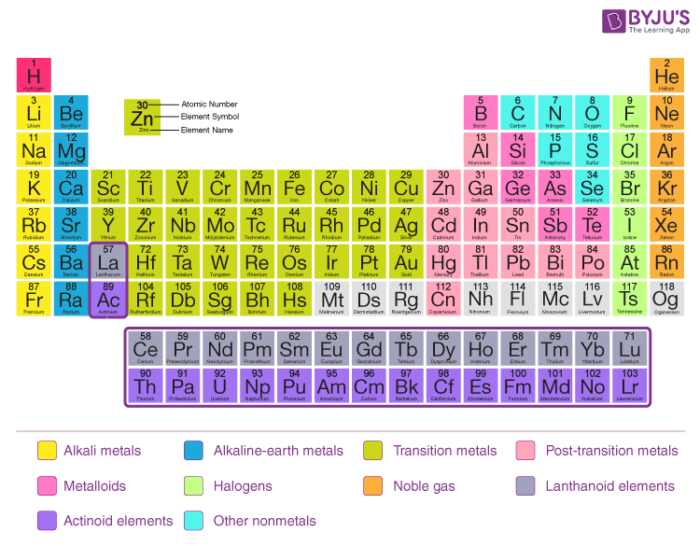
In the long form periodic table the elements are arranged in the order of their atomic numbers. Atomic number of an element is equal to the number of protons inside the nucleus of its atom.
The general features of the long form periodic table are:
- There are in all, 18 vertical columns and 18 groups in the long form periodic table.
- These groups are numbered from 1 to 18 starting from the left.
- There are seven horizontal rows called periods in the long form periodic table. Thus, there are seven periods in the long form periodic table.
- The elements of Groups 1, 2 and 13 to 17 are called the main group elements. These are also called typical or representative or normal elements.
- The elements of Groups 3 to 12 are called transition elements.
- Elements with atomic number 58 to 71 (Ce to Lu) occurring after lanthanum (La) are called lanthanides. Elements with atomic numbers 90 to 103 (Th to Lw) are called actinides. These elements are called f-block elements and also as inner transition elements.
Today we study the elements with the help of the modern periodic table. Periodic classification of elements is the method by which elements are grouped on the basis of their characteristics i.e. we keep the elements that are alike in one group and the rest of the elements in the other group. Some empty spaces have been left in the periodic table so that the elements that will be discovered in the future can be placed without disturbing the trending periodicity of the elements.
Check ⇒ History of Periodic Table
Recommended Videos

Significance of the Periodic Classification of Elements
- Makes the study of elements easy: Classification of elements in groups provide us with a fixed pattern in which the elements change their properties periodically. The periodic table made the study of the physical and chemical properties of elements simple and organized. We can now just go to the group and see the properties of the elements of the periodic table or predict the properties of an element if we know the characteristics of other elements present in the same group.
- Helps in discovering new elements: Although there are so many elements that are already discovered, there are chances that new elements can be discovered. Scientists can take the help of a periodic table and know the trending characteristics based on the properties of elements and hence can identify the new elements with the existing ones. Besides, researchers are continuously striving to discover new elements and explore their properties.
Frequently Asked Questions – FAQs
What was the need of classification of elements?
Grouping the elements into different classes is called periodic element classification. This approach involves the arrangement of related elements, and the separation of unlike elements. Comparison of the properties of elements. It helps us to understand how different compounds make up different elements.
What were the limitations of dobereiner classification?
Only three triads could Dobereiner find; I.e a total of only 9 elements. The total number of elements, however, was more than that of those included in the Triad of Dobereiner. Thus, most of the elements known at that time could not be classified by the Dobereiner's.
What are the advantages of classification of elements?
It allows chemists to predict the properties of the elements and their compounds in the periodic table based on their positions, and vice versa. Studying, understanding, comparing and contrasting the relative properties between the elements and their compounds from different groups becomes easier.
What elements are in the main group?
In chemistry and atomic physics the main group is the group of elements (sometimes referred to as representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon, nitrogen , oxygen, and fluorine as arranged in the periodic table of elements.
What are the two types of alloys?
There are 2 major alloy types. These are called interstitial alloys and substitutional alloys. In substitute alloys, the original metal's atoms are essentially substituted by atoms that have approximately the same size from another element. For example, brass is an example of a copper and zinc replacement alloy.
Now get NCERT Solutions for the chapter Classification of Elements and Periodicity and explore in-depth about the concept at BYJU'S. You can also download BYJU'S – The Learning App to know more about periodic table groups, modern periodic law, different elements in Chemistry, and more.
swansonsumpeormses.blogspot.com
Source: https://byjus.com/chemistry/classification-of-elements-in-modern-periodic-table/
Post a Comment for "Section 2 Classification of the Elements Continued"